- 02 november 2022
- Door Mels Dees
How to construct collagen scaffolds
Designing and building collagen-based superstructures is an essential condition for the development of both medical practice and lab research. Surprisingly, systematic research has been scarce. At TU/e (Eindhoven University of Technology) Laura van Hazendonk carried out her graduation research into the engineering of multiscale collagen scaffolds, working together with PhD student Paula Vena and Assistant Professor Heiner Friedrich. With her work, she won the 2020 AkzoNobel Graduation Award for Chemistry and process Technology.
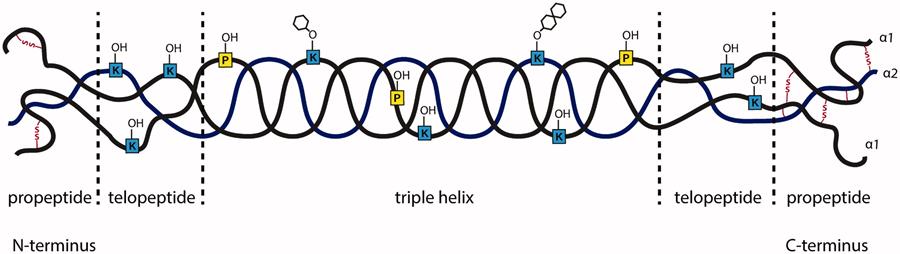
Engineering scaffolds
The trillions of cells making up ourselves and other mammals, are – almost all of them – organised in matrices of collagen, the most abundant protein in our bodies (25% to 35%). Collagen consists of amino acid polymers bound together to form elongated fibrils. It is found mostly in connective tissue such as cartilage, bones, tendons, ligaments, and skin.
Apart from its task as a glue, as the name suggests, collagens play widely varying roles. Depending upon the degree of mineralization, collagen tissues may be rigid (bone), compliant (tendon) or something in between (cartilage). In vivo, the organising matrix is determined by its function, to provide support and a fitting environment for the cells.
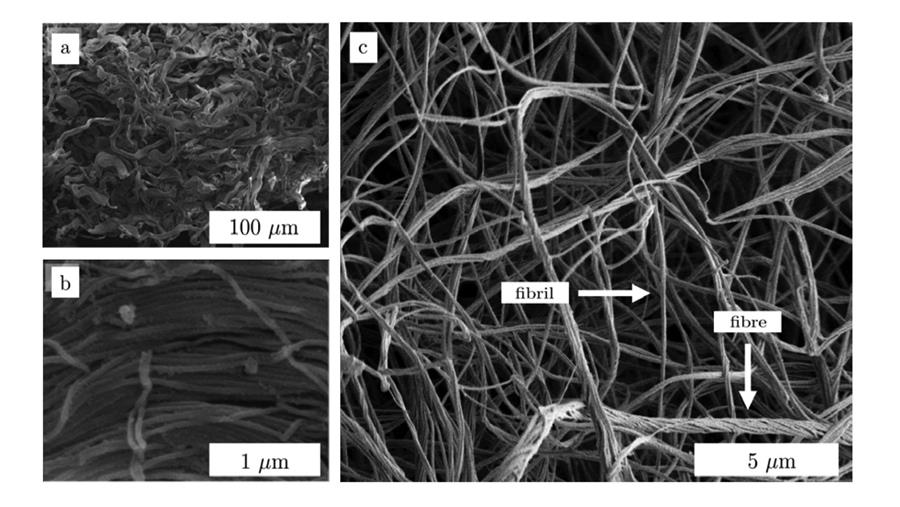
Therefore, if we want to create or modify living tissues for medical purposes – tissue regeneration or reconstructive surgery, for instance – we need to create purpose-built collagen scaffolds. However, a handbook for collagen scaffold building does not (yet) exist. Past research and experiments of methods and conditions often seems contradictory and insufficient.
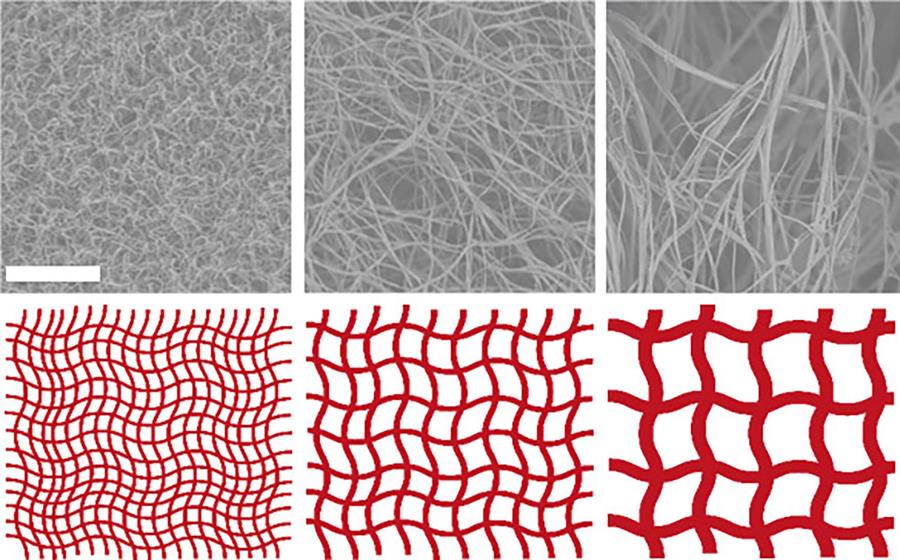

About collagen
Paula started by explaining the nature of collagen: “In all, there are about 28 different types of collagen in our body. Collagen type I is the most abundant and therefore we use it in the lab as a scaffold to grow cells and study processes in vitro. At the moment collagen is sometimes injected in injured tissue as a gel, but we hope to be able to mineralize it, for instance with calcium phosphate, and use it to repair or replace bone structures and other tissues.”
"We had to measure a lot of process conditions at the same time, so we had to design an efficient workflow"
Of course, collagen is also widely sold commercially – for instance in the cosmetic industry. "You can get collagen drinks, powders etc. But most scientists have serious doubts about the effectiveness.”
To which Heiner Friedrich added: “It probably has all of the effect of a handful of jelly beans!”
Top-down and bottom-up
“The most important thing now is that we find a systematic way to create scaffolds, Paula says. “In our research we worked in two ways: top-down by creating structures using 3D-printing, and bottom-up by influencing the way the superstructures develops.”
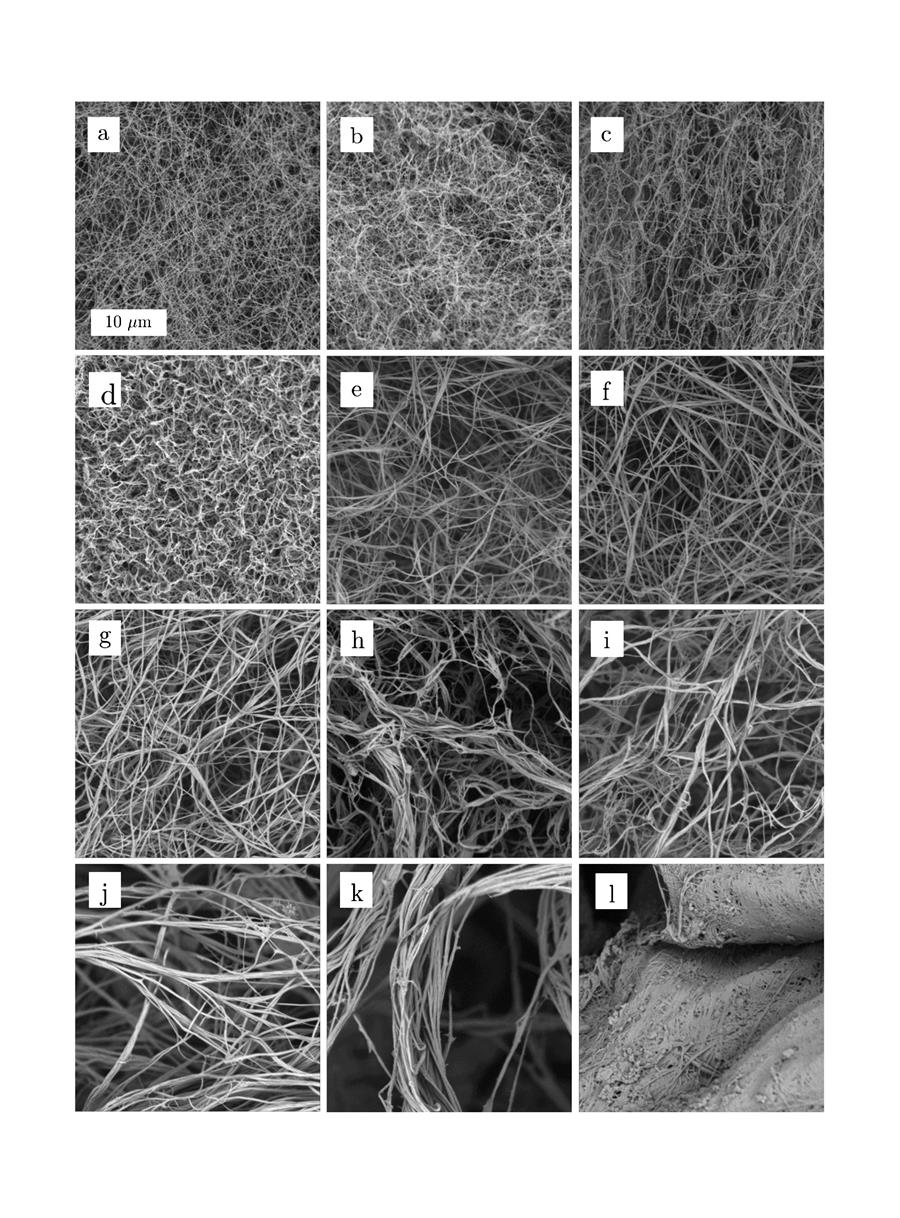
Laura explains: “By changing the physical environment in which the collagen molecules are formed or self-assemble – in your body or in the lab – the shape of the fibres will be altered. It is determined by factors such as pH, salt concentration and temperature. And collagen scaffolds are structured on many different levels and scales. Starting with polymerised protein molecules that organise into tiny fibrils, from which larger-scaled fibres are formed. These again combine into fibre bundles from which the scaffolds are made. However, the definitions are not very consistent in scientific literature.”
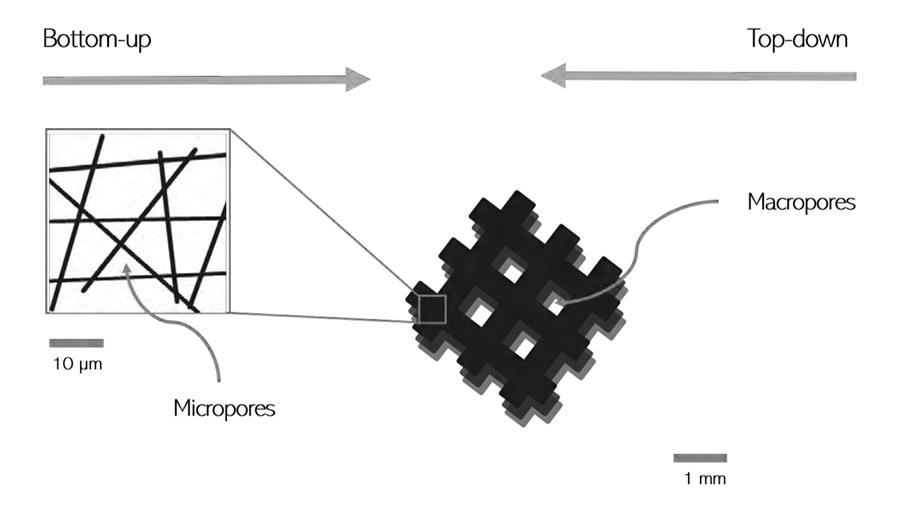
Gaining control
Paula: “What is interesting, is that fibres will not combine into bundles under all conditions. In different organs they form differently shaped networks in our body. We are still far from achieving that in a lab, though.”
In vitro, the beginning is pretty easy: you make a collagen solution, increase the pH, increase the temperature, maybe add a little salt and you end up with fibres. In the body, the form these fibres take is determined by other peptides and many other factors we cannot reproduce, at least not yet.
“And because there was no systematic study available,” Paula adds, “Laura set up this research project. In the first place the challenge was to control the fibre dimensions, in particular the diameter, in vitro. We tried to keep it as simple as possible: with the minimal number of variables to manipulate, and using just a few salts, such as sodium phosphate and sodium chloride.”
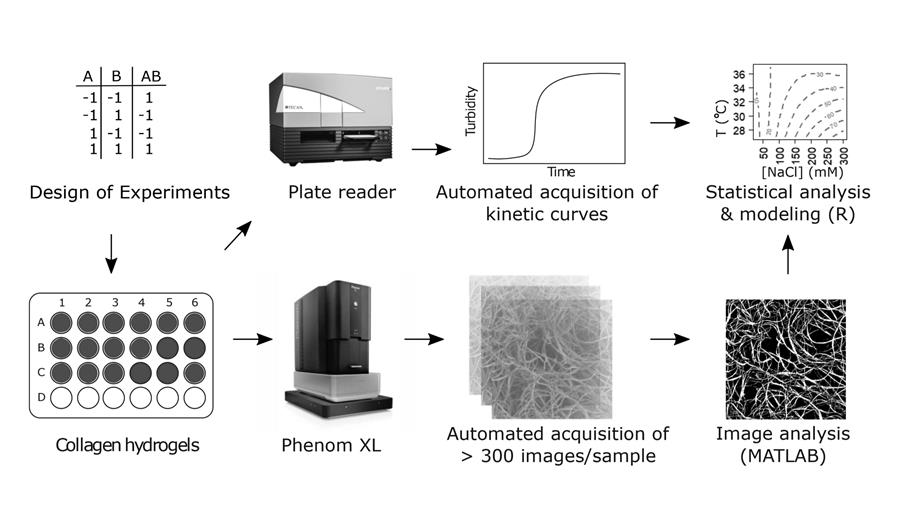
design of an efficient Workflow
Laura explains that, instead of ‘just trying something’, as had often been done in the past, they wanted to make a systematic analysis of the process. “For that, we had to measure a lot of process conditions at the same time – if we were to take just one of the factors and keep the others constant, the research would take years. However, all these measurements together would create an enormous amount of data to be processed, so we had to design an efficient workflow.” They chose a combination of smart statistical sampling to reduce the number of experiments and get the most information out of them, combined with automated image analysis of both kinetic processes and dried material.
“In the end,” Laura says, “we wanted answers to a series of fundamental biochemical questions, but the methods we chose were more in an engineering tradition. We felt at home at TU/e and that helped a lot.”
Different approaches
Paula adds to that: “What I learned from Laura’s work is that basically, you have two types of questions: one in which you hardly have a clue what will come out in a qualitative sense – and the other kind, in which you try to determine the quantitative balance between various factors that are determined from the outset. These are extremes, of course, and usually you have both types of questions in a project, but they require different approaches. I have become very much aware of that. In this case, Laura’s approach worked very well indeed. And I am already applying this knowledge in my present research.”
Heiner Friedrich recognized the different approaches. “Notwithstanding,” he said: “at the moment, there is a general trend in science and engineering, where these approaches are coming together. The most common approach is to have automated experimentation, take the resulting, enormous amount of data, and then mine them for predictive models. But Laura designed the experiments – from an engineering perspective – while she was already thinking of the way the results could be mined afterwards. A step forward, if you ask me.”
successful Teamwork
Indeed, the resulting research has proven successful, as was the team’s cooperation. All would look forward to working together on a continuation of the project. Laura: “And the idea of doing something thoroughly technical while at the same time improving other people’s lives appeals to us all.”
